Description
N-Allyloxycarbonyl (Alloc) are widely used protecting groups, i.e. for amines and amino acids, as they are quantitatively and very rapidly converted to free amino compounds by palladium. Deprotection of allyl (All) carboxylates and allyl aryl ethers can also be carried out accordingly. All and Alloc are fully compatible with Fmoc/tBu and Boc/Bzl strategies, as well as with Z protecting groups. References: Highly efficient removal of allyloxycarbonyl (Alloc) function provides a practical orthogonal protective strategy for carbohydrates; Guang Hui Zong, Shi Qiang Yan, Xiao Mei Liang, Jian Jun Zhang, Dao Quan Wang, Fan Zuo Kong; Chinese Chemical Letters 2009; 20: 127-130; DOI:10.1016/j.cclet.2008.11.002. Mild non-transition metal catalyzed deprotection of N-allyloxycarbonyl amines; Ronald H. Szumigala, Jr., Ekama Onofiok, Sandor Karady, Joseph D. Armstrong III, and Ross A. Miller; Tetrahedron Letters 2005; 46: 4403-4405. DOI: 10.1016/j.tetlet.2005.04.064. Allylic Protecting Groups and Their Use in a Complex Environment. Part II: Allylic Protecting Groups and their Removal through Catalytic Palladium p-Allyl Methodology; François Guibé; Tetrahedron 1998; 54: 2967-3042. Selective Cleavage of the Allyl and Allyloxycarbonyl Groups through Palladium-Catalyzed Hydrostannolysis with Tributyltin Hydride. Application to the Selective Protection-Deprotection of Amino Acid Derivatives and in Peptide Synthesis; O. Dangles, F. Guibé, G. Balavoine; S. Lavielle, A. Marquet; J. Org. Chem. 1987; 52(22): 4984-4993.
x
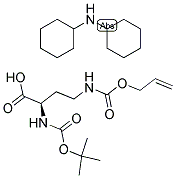
Alternative Distributors of [N-alpha-t-Butyloxycarbonyl-N-gamma-allyloxycarbonyl-D-2,4-diaminobutyric acid dicyclohexylamine]
Producers or manufacturers change the product range from time to time.
The following companies
have appeared as suppliers in the past / currently not verified
Alabiochem Tech. Co., Ltd. | Shanghai Hanhong Chemical Co., Ltd. | LaborMedizinHandel Zwickau GmbH


 Chemical-Suppliers.eu
Chemical-Suppliers.eu