Categories
IntermediatesMetabolites
Impurities
Carbohydrates
Aromatics
Glucuronides
Oligosaccharides
Nucleotides
Flavonoids
Oils
Heterocycles
Aliphatics
Phytochemicals
Inhibitors
Catalysts
Anthocyans
Triterpenoids
Amines
Saponins
Neurochemicals
Carotenoids
Cardioglycosides
Inositols
Activators
Iridoids
Retinoids
Dihydropyridine
Sesquiterpenoids
Detergents
Peptides
Frequently visited
Hydrogen peroxideParaffin wax
Helium
Salicylic acid
Isopropyl alcohol
Potassium nitrate
Urea 46%
Salicylic acid
Sodium bicarbonate
Ethyl alcohol
Chloroform
Sodium hydroxide
Carbon activated
Vitamin B12
Sulfuric acid
Hydrochloric acid
Propylene glycol
Boric acid
Resins
Polypropylene
Nitric acid
Iron
Copper
Glycerine
Propylene glycol
Sodium chlorite
Polyurethane
Phosphoric acid
Melamine
Acetic acid
Copyright © 2002 - 2024 Chemical-Suppliers.eu . All rights reserved.

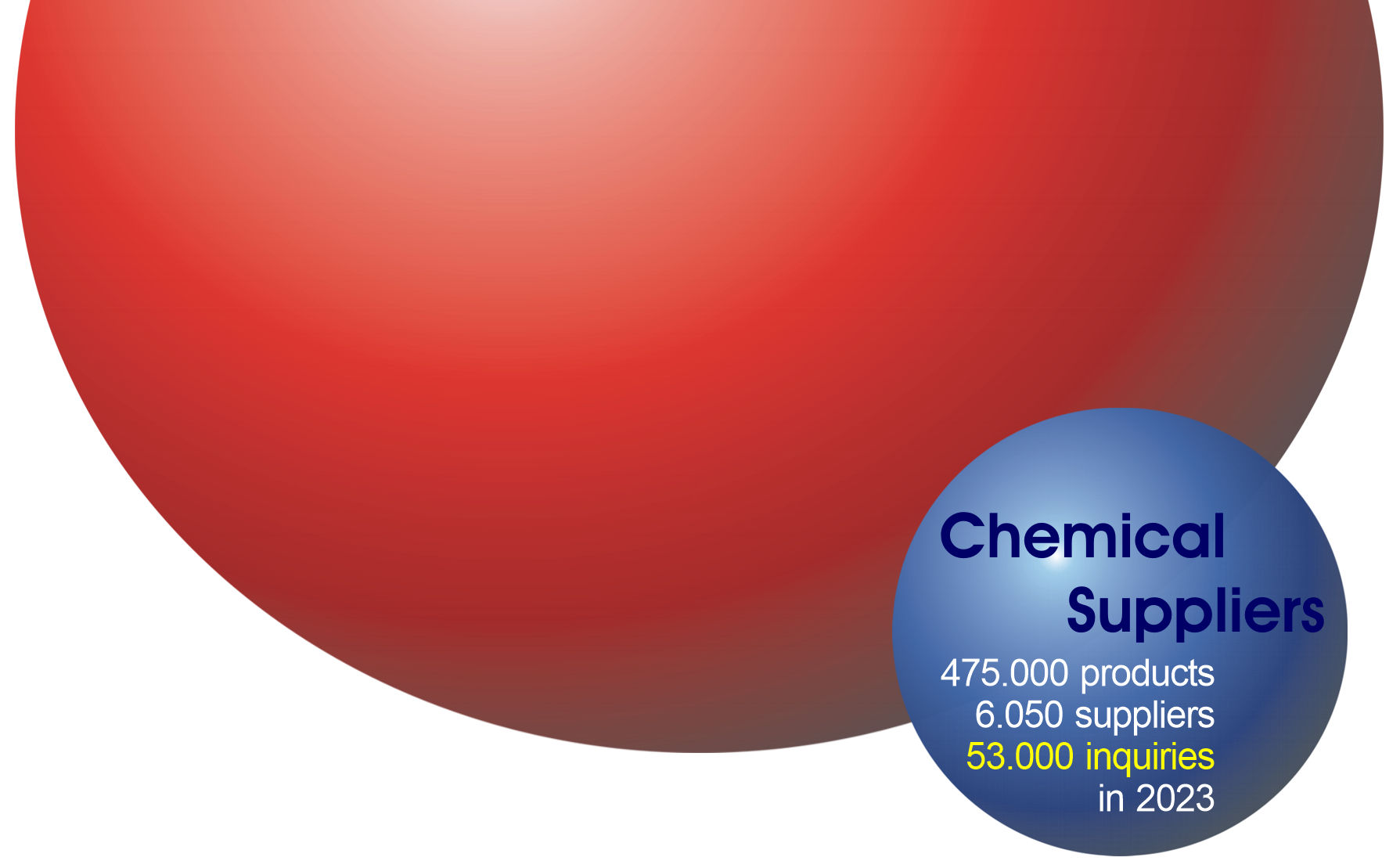

 Chemical-Suppliers.eu
Chemical-Suppliers.eu