x
Safety & Transport Information
A poison gas which forms methemoglobin in the body and destroys red cells causing anemia, anorexia, and cyanosis. Recovery is said to be rapid, leaving no permanent physiological damage. Can be absorbed through the skin. Its odor can be detected as low as 10 ppm although this cannot be relied upon as an indication of toxic concentration in air. While non-flammable, it supports combustion. It is a powerful oxidizer. Moderately explosive. Potentially explosive reactions with combustible gases or vapors. benzene + aluminum trichloride, benzocyclobutene + butyllithium + potassium tert-butoxide, calcium acetylide, potassium cyanide, potassium thiocyanate, sodium iodide, charcoal, ethyl-4-fluorobenzoylacetate, hydrocarbons, hydrogen sulfide, nitrogen oxide, sulfur dichloride, vinylidene chloride, 3α-hydroxy-5β-androstane-11,17-dione-17-hydrazone, lithiated compounds, 2-lithio(dimethylamino-methyl)ferroxene, methyl-2-bromo-5,5-ethylene dioxy(2,2,1)bicycloheptane-7-carboxylate, aliphatic heterocyclic amines, sodium methoxide + methanol, vinylidene chloride. Reacts to form explosive products with nitrogenous bases (e.g., isopropylamine, isobutylamine, aniline, phenyl hydrazine, 1,2-diphenyl hydrazine), sawdust, lampblack. Violent reaction with finely divided organic materials. A fluorinating agent in chemical synthesis, and an oxidant in rocket fuel. When heated to decomposition it emits toxic fumes of F− and Cl−. See also FLUORINE and PERCHLORATES.
Hazard Symbols:
Dangerous in contact with organic materials, strong oxidizing agent. Toxic by inhalation. TLV: 3 ppm; STEL 6 ppm.
RID/ADR certification :
3157
x
Alternative Distributors of [Perchloryl fluoride]
Producers or manufacturers change the product range from time to time.
The following companies
have appeared as suppliers in the past / currently not verified
BOC Sciences

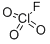

 Chemical-Suppliers.eu
Chemical-Suppliers.eu