x
Hazard Statements
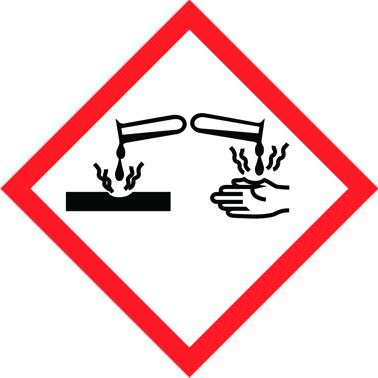
GHS05
Corrosive
- Corrosive to metals, category 1
Safety & Transport Information
Moderately toxic by intraperitoneal route. A very reactive alkali metal (more reactive than potassium or cesium). In the body, rubidium substitutes for potassium as an intracellular ion. The ratio of Rb/K intake is important in the toxicology of rubidium. A ratio above 40% is dangerous. In rats, a failure to gain weight is the first symptom, followed by ataxia and hyperirritability. Symptoms include: skin ulcers, poor hair coat, sensitivity, and extreme nervousness leading to convulsions and death.A very dangerous fire and explosion hazard when exposed to heat or flame or by chemical reaction with oxidizers. Ignites on contact with air, oxygen, and halogens. Ignites spontaneously on contact with water. Reaction with water, moisture, or steam forms explosive hydrogen gas, which then ignites. Explodes in contact with liquid bromine. Can react explosively with air, halogens, mercury, nonmetals, vanadium chloride oxide, moisture, acids, oxidizers. Violent reaction with vanadium trichloride oxide (at 60°C), Cl2O2, P. Molten rubidium ignites in sulfur vapor and reacts vigorously with carbon. RbOH is more basic than KOH. Storage and handling: Keep under benzene, petroleum, or other liquids not containing gaseous O2. When heated to decomposition it emits toxic fumes of Rb2O. See also SODIUM and POTASSIUM SODIUM ALLOY.
Risk Codes:
R14/15 - Reacts violently with water, liberating extremely flammable gases
R34 - Causes burns
R36/37/38 - Irritating to eyes, respiratory system and skin
R36/38 - Irritating to eyes and skin
Safety Codes:
S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36 - Wear suitable protective clothing
S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection
S43 - In case of fire use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water)
S45 - In case of accident or if you feel unwell seek medical advice immediately (show the label where possible)
Hazard Symbols:
Reacts vigorously with air and water, must be stored under kerosene or similar liquid, dangerous fire and explosion risk. Metal causes serious skin burns.
RID/ADR certification :
UN 2031 8 / PG 2
x
Alternative Distributors of [Rubidium, 99.75% (metals basis)]
Producers or manufacturers change the product range from time to time.
The following companies
have appeared as suppliers in the past / currently not verified
Reade Advanced Materials | ABCR GmbH & Co. KG | C-LAB Chemievermittlung GmbH




 Chemical-Suppliers.eu
Chemical-Suppliers.eu